Electronic Configuration of Magnesium Cation is explained here. The electronic configuration gives insight into how electrons are arranged or distributed across a molecule or atom. This arrangement is guided by the Aufbau principle, which states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. According to the Aufbau principle, the available atomic orbitals with the lowest energy levels are occupied before those with higher energy levels. The number of electrons a shell or orbital can hold is based on ‘The Pauli exclusion principle. To understand any element’s electron configuration, we need first to have a basic understanding of the element and its properties.
Magnesium
Magnesium (Mg) is a naturally occurring Alkaline earth metal which belongs to group 2 (IIa) of the periodic table. It is silvery-white and lightweight. Its relative density is 1,74 and it’s density 1740 kg/m3 (0.063 lb/in3 or 108.6 lb/ft3). Its low weight is known as the best form of metal for forming mechanically resistant alloys. It is a stable and neutral element with no Positive charge ( +) or Negative charge (-) attached to it: the number of protons equals the number of electrons, which gives it its stable state. Its cation, Mg2+, is also essential in almost every biochemical metabolism.
Properties of Magnesium (Mg)
State: Solid
Atomic Number: 12
Atomic Weight: 24.305
Electronic Configuration: [Ne]3s² (1s² 2s² 2p6 3s2)
When binding with other elements (usually non-metals), Group IIa elements, i.e., alkaline earth metals such as Magnesium, are characterized by their tendency to lose or gain two electrons to form a type of bond called ‘Ionic Bond. When an element loses an electron to bind with a non-metallic element, the bond formed is called a cationic bond, and the element forms a Cationic element. When it gains 2 or more electrons, it is called an Anion. :2+ ‘cation’ i.e Magnesium cation (Mg2+).
Mg—> Mg²+ + 2e-
Magnesium Cation Mg2+
Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital.
Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape. Mg2+ has an electronic configuration of 1s² 2s² 2p^6, i.e., has a total number of 10 electrons similar to that of a Noble gas [Ne] instead of Mg 12 as its total number of electrons and configuration of 1s² 2s² 2p6 3s².
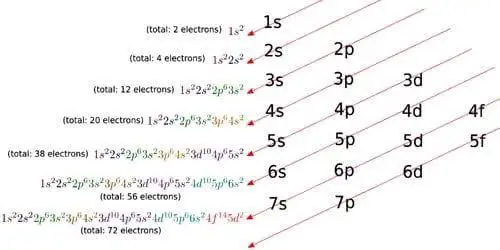
Order of Electron Arrangement
n, The diagram below shows us how electrons are arranged around each shell or orbital and how many each shell can ca to understand this shift in quantum energy level or loss of electrons. The Aufbau Principle guides this principle of arrangement
The Periodic Table can also help us better understand this.
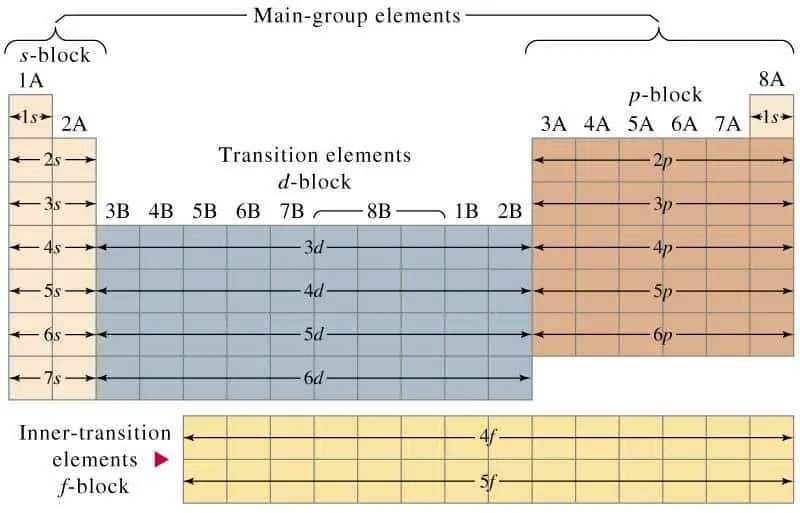
n stands for the shell number
shell 1 has a sub-shell called the s subshell which can only carry a maximum number of 2 electrons
Shell 2 has the subshells s and p
Shell 3 has subshells s, p and d
Sub-shell carries a maximum number of 2 electrons, p always carries a maximum of 6 electrons. and d carries a maximum of 10 electrons.
How electrons are distributed across Magnesium(Mg) and (Mg2+)
orbital: 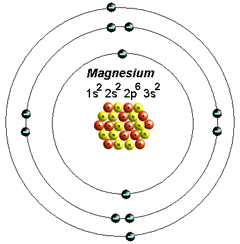
1st shell = 1s2
2nd shell = 2s2 , 2p6
3rd shell = 3s2
For Magnesium cation (Mg2+) the electronic configuration involves removal or loss of electrons from Magnesium’s valency shell, which it then attains.
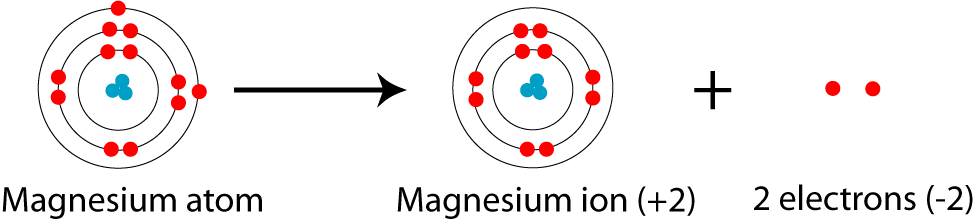
1s²2s²2p^6 3s² —-> 1s²2s²2p^6. + 2e-
Properties of Mg2+
State: Solid
Atomic Number: 12
Electron: 10
Atomic weight: 24.305
Electronic Configuration: 1s² 2s² 2p6
