Mass of Hydrogen in Kg? Hydrogen’s molecular weight is 1 amu, equal to 1.66×10−27 Kg, as it has only 1 proton and no neutron, so it is 1 atomic unit.
Summary
Hydrogen is the lightest element in the periodic table and the most abundant substance, which comprises almost 75% baryonic mass. Hydrogen’s molecular weight is 1 amu, equal to 1.66×10−27 Kg, as it has only 1 proton and no neutron, so it is 1 atomic unit.
Main Article
The atomic weight of a molecule depending on the number of neutrons and protons available in the element. The weight of 1 neutron is equal to 1 proton, so it is important to find the number of elements, and then, accordingly, atomic weight can be identified. These particles play a major role in defining many features of the element, which is not restricted to weight. They also define its atomic number, position in the periodic table, its characteristics, etc.
Hydrogen is the first element that has only 1 proton and no neutron due to which its atomic mass is 1 amu.
1 amu = 1.66×10−27 Kg
The relationship of unified atomic mass unit to macroscopic SI base unit of mass, the kilogram, is given by Avogadro’s number NA.
By definition, Avogadro’s number – the mass of NA carbon-12 atoms at rest and ground state is 12 g ( = 12×10−3 kg). The latest value of NA, the unified atomic mass unit:
1 amu ≈ 1.660 538 782(83) × 10−27 kg
The unit u is easy to use and popular because one hydrogen atom has a mass of approximately 1 u,
( as it has only 1 proton).
Hydrogen is the lightest element available, which many other properties like colorless, odorless, and highly frameable ability. It burns with pale blue color, which is almost invisible in many cases. Its flammable nature is stored in a secure location and specialized containers as prolonged exposure to fire and heat and lead to rupture, which will be like a rocket launch explosion and highly dangerous for people and the environment nearby. Hydrogen is non-toxic normally but can act as an asphyxiant by replacing all air and creating damage. It has the second-lowest boiling points than helium.
The boiling point for hydrogen – 20 K (–423 oF; –253 oC)
The melting point for hydrogen – 14 K (–434 oF; –259 oC)
These properties help in storage, transportation and improve its usability as it can be stored in gas and liquid form.
The basic chemical property of Hydrogen is mentioned below:
| Property | Value |
| Molecular Weight (g) | 2.016 g/mol |
| Exact Mass (g) | 2.01565 g/mol |
| Monoisotopic Mass (g) | 2.01565 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 0 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Molecular Formula | H2 |
Density of hydrogen in different forms:
| Substance | Vapor Density (at 68 oF; 20 oC, 1 atm) | Liquid Density (at normal boiling point, 1 atm) |
| Hydrogen | 0.005229 lb/ft3 (0.08376 kg/m3) | 0.0406 lb/ft3 (0.65 kg/m3) |
Hydrogen structure is single, and it has one single bond. Both the atoms are connected and make a stable structure of H2. (mentioned below). This has many electron donor features, metabolites for the human body, used in food packaging industries, etc.
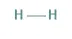
The usability of hydrogen for commercial purposes due to its flexibility, lightweight is mentioned below:
- Hydrogen work as an energy vector for vehicles:- As a cleaner and advance fuel alternative, it is used in many different types of vehicles like cars, trucks, two-wheelers, etc. They are also used in combination with electricity batteries as a backup. So that users can switch between them and travel more distance in one go without re-fueling or charging.
- Hydrogen is cryogenic fuel for various types of machines, tools, and the food industry. It has helped create a whole new world in all the sectors for innovation and creativity, which was earlier not possible.
- Hydrogen property to get liquify and remain stable under a controlled environment has helped design safe and secure supply chain methods that make it available commercially.
- Compressed hydrogen flexibility has helped use the same in fuel cells, which can work as an electrical power source for anything possible. Also, it is very light in weight and takes less space than many other options available.
- Hydrogen is a flexible component in the chemical industry, both in fuel and as a component in chemical reactions. In many industries, it is due largely to both key ingredient and by-product simultaneously, which helps save cost and make it effective.
Nowadays, there is a number of the commercial Hydrogen production process by which it is can be commercially made available to everyone. All the processes mentioned below are critical and should be done in a clean and controlled environment. All the required safety mechanisms as hydrogen are a very flammable and dangerous element and can harm many.
- Production of hydrogen by reaction of aluminum and water in the presence of NAOH and KOH. This is a stable and efficient process of producing hydrogen and also amount produced in huge.
- The steam methane process is also used for the production of hydrogen on a large scale. In this, methane is used to separate carbon from hydrogen and get the ultimate required product.
- In this electricity and electrodes, hydrogen production’s electrolysis process is used to separate hydrogen elements and generate them in good quantity.
- Many thermochemical processes are also used to generate hydrogen from water, to generate oxygen from a water molecule, and get dihydrogen or hydrogen as output depending on the design of the system and also on the requirement for which it will be used like it is needed for storage and transportation or will be used there itself in other processes.
- The steam methane reforming process is also used as a generic process, but it is a costly process and needs extra precautions while it’s going on.
Ultimately packing is one of the key steps in using hydrogen as a fuel or element in many places. So it needs to be either taken as a liquid in pressurized cylinders or needs to be stored in the gas state, which is leak-proof, and maintained properly.
Reference:
https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen
https://www1.eere.energy.gov/hydrogenandfuelcells/tech_validation/pdfs/fcm01r0.pdf
https://www.angelo.edu/faculty/kboudrea/periodic/structure_mass.htm
https://www.sciencedirect.com/topics/engineering/compressed-hydrogen

